We can and
Together we will
Our mission is to eliminate the challenges of Prader-Willi Syndrome (PWS) through research and therapeutic development.
How We Help
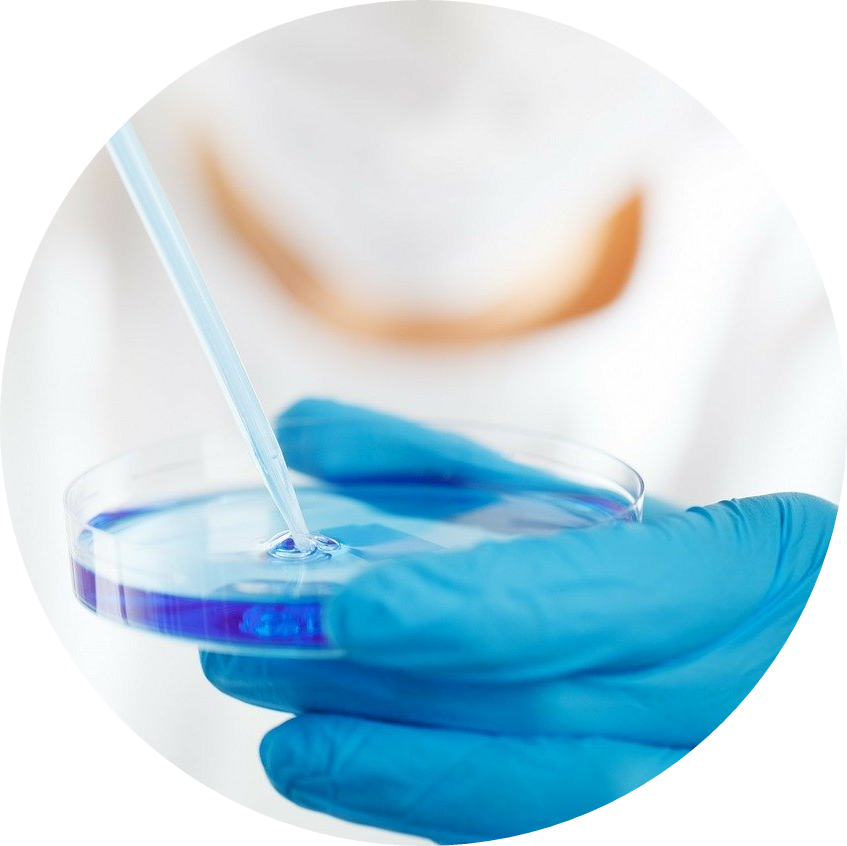
Research is key!
FPWR UK raises money for the sole purpose of research. Research will help us to improve the lives of individuals with PWS

How Everyone Helps

Fundraise!
There are many ways to get involved:
- One Small Step Walk
- Donate
- Run
- Much more

How You Help

Global Registry
By participating, individuals and families of those with PWS become part of the research team, helping uncover trends which inform new directions in therapies and treatment.
Meet PeeWee
PeeWee is our FPWR UK mascot and supports our One Small Step Walks and other fundraising events. He travels all over the world to make new research discoveries, raise money to invest in research and have fun!
Help PeeWee make new research discoveries by Donating Today or Fundraising for FPWR UK

Mission Statement
To eliminate the challenges of Prader-Willi Syndrome (PWS) through the advancement of research.





