Study Purpose
KITE PWS is a Phase 2 study to investigate the effects of RGH-706 in people with Prader-Willi Syndrome. This study is seeking people aged 17 years and older with Prader-Willi syndrome for participation and will evaluate an experimental oral drug that may help to reduce appetite. The study lasts 5-6 months and requires about 8 study visits.
The purpose of this study is to learn more about an experimental drug called RGH-706. RGH-706 is an experimental oral drug that blocks melanin-concentrating hormone (MCH), which is a key part of the brain’s food-seeking mechanism. When this hormone attaches to specific receptors in the brain (i.e., proteins on the outside of brain cells), it increases the desire to eat. RGH-706 may help to block this hormone and reduce appetite.
The study drug, clinic visits, and study-related procedures are provided at no cost. Participants and caregivers may also receive reimbursement for travel and meals.
Recruitment Criteria
Study Type: Interventional
Eligible Ages: 17 years and older
Other Criteria:
To be in the study, participants must meet the following basic criteria:
- Are at least 17 years of age
- Have a diagnosis of PWS
- Have a body weight of greater than 40 kg (88 lbs.) and less than 200 kg (450 lbs.)
- Have had a stable body weight for the past 3 months
- Have at least 1 consistent and reliable primary caregiver who can evaluate changes in the participant’s hyperphagia symptoms, mood, health, and behavior throughout the study
- Do not have uncontrolled diabetes or diabetes that requires insulin
Medical history and other criteria will also be reviewed to determine eligibility. See the trial listing at clinicaltrials.gov for a full list of inclusion and exclusion criteria.
Trial Details
ClinicalTrials.Gov Id: NCT05322096
Phase: 2
Duration: 5-6 months with 8 study visits
Lead Sponsor: Gedeon Richter
Countries: United States, Czechia, France, Italy, Spain
Learn more about this study by watching this short presentation: 'What can I expect if I participate in Kite-PWS?'
This study will be available at the following sites:
Multiple sites are available in the United States, Czechia, France, Italy, Spain.
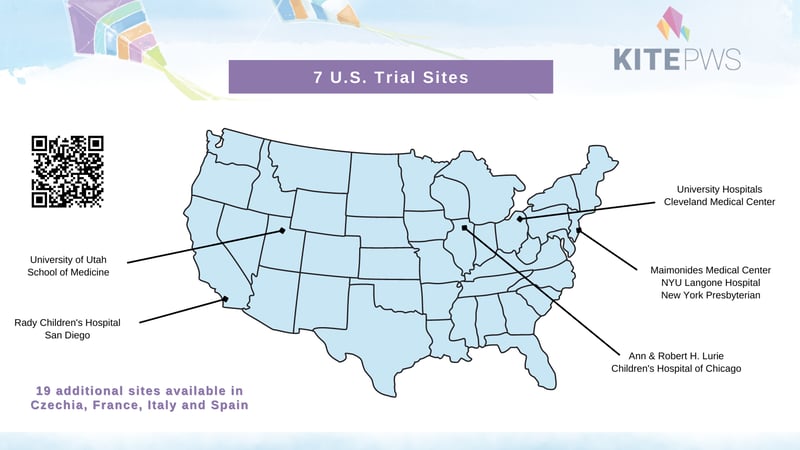
CALIFORNIA
San Diego Rady Children's Hospital
e-mail: hfolk1@rchsd.org; lbird@rchsd.org
phone: (858) 576-1700 ext. 221543; (858) 966-5808
NEW YORK
New York Morgan Stanley Children's Hospital of NewYork
e-mail: jc688@cumc.columbia.edu
phone: (212) 305-5508
Mineola NYU Langone Hospital—Long Island
e-mail: Jorge.Mejia-Corletto@nyulangone.org marilyn.richardson@nyulangone.org
phone: (516) 663-351
Brooklyn Maimonides Medical Center
e-mail: DEsingh@maimonidesmed.org; hmustafic@mainmonidesmed.org
phone: (718) 283-8160
OHIO
Cleveland University Hospitals Medical Center
e-mail: genya.kisin@uhhospitals.org; Jirat.Chenbhanich@UHhospitals.org
phone: (216) 286-9202
ILLINOIS
Chicago Ann & Robert H. Lurie Children's Hospital of Chicago email: PKhanna@luriechildrens.org; sratnam@luriechildrens.org
phone: (312) 227-6090
Visit ClinicalTrials.Gov the most current list of sites.
Questions?
Contact: Medical Information Scientific Service
Phone: +36 1 505 7032
Email: medinfo@richter.hu




