
Perhaps now, more than ever before, we are beginning to see the fruits of more than a decade of advocacy work. With your support, FPWR has been working diligently to build a "research-ready" community, with resources that address the FDA’s call for patient experience data, demonstrate the high disease burden of PWS and establish the need for effective therapies. Our ultimate goal: the successful approval of new treatments for PWS.
Advocating for new treatments for PWS needs to happen all the way across the drug development and approval pathway in order to achieve optimal effect. Such an approach will ensure that new treatments developed for PWS will have a meaningful impact on our loved ones. This means working with pharmaceutical companies to help them understand the medical needs of the PWS community and the aspects of PWS that families want to see treated. It also requires establishing a strong relationship with the Food & Drug Administration (FDA), whose job it is to determine if a drug is safe and effective for the PWS. The FDA does this by weighing the benefits and risks of any new drug, in the context of the unmet medical needs of the patient population who will potentially be taking the drug.
Regulatory scientists at the FDA have to review treatments for many, many different disorders so they seek input from the patient community about what the medical needs of patients are, what a meaningful outcome would be, and what level of risk individuals are willing to accept for the potential benefit they might receive from a treatment. With the 21st Century Cures Act, the opportunities for patients to provide input to FDA were strengthened and formalized, and the FDA has established programs to encourage Patient-Focused Drug Development.
The FPWR team has been working to gather information about the PWS patient and family experience and preferences to share with pharmaceutical companies and the FDA to help accelerate the development of meaningful new treatments for PWS. Our efforts include:
.png?width=900&name=PWS%20Advocacy%20Activities%20(7).png)
For more than a decade the FPWR has partnered with families and other PWS groups (PWSA|USA, IPWSO) and worked diligently to build resources that address the FDA’s call for patient experience data, demonstrating high disease burden and the need for effective therapies addressing hyperphagia. We have demonstrated through family stories, surveys, and a more formal, best-worst scaling study that hyperphagia is “the” aspect of PWS that families want addressed through new therapies, followed by other critical behavioral issues such as anxiety and aggression.
We have demonstrated the tremendous unmet medical needs of individuals with PWS, and the considerable burden of disease, showing that caregiver burden in PWS exceeds that of caregivers for stroke and Alzheimer disease (Kayadjanian et al 2018) and that higher levels of hyperphagia are associated with increased caregiver burden (Kayadjania et al 2021).
We have also demonstrated that caregivers are willing to accept considerable risk in exchange for modest improvements in hyperphagia (Tsai 2021).
Finally, as suggested by the FDA during a Critical Path Innovation Meeting, we have also solicited input from individuals with PWS, speaking on their own behalf. Although this was challenging (not all individuals are capable of expressing their views), Dr. Elizabeth Dykens and her team elicited opinions from young adults with PWS on treatment preferences and disease burden, which strongly align with that of caregivers (Dykens 2021). This patient experience data is complemented by natural history studies that provide critical information to support clinical trials, including a study funded by the National Institutes of Health (led by Dr. Dan Driscoll, 2006-2014) as well as an ongoing registry-based prospective study of behavioral changes over time and serious medical events (PATH for PWS).
The 143-page letter included more than 26,000 signatures from community supporters around the globe and comments from trial participants and PWS community members.
FPWR has regular interaction with pharmaceutical companies who may have an interest in developing new treatments for PWS, with the aim of educating the companies about PWS and helping them develop successful clinical trials that minimize burden on participants. Our goal is to provide the patient perspective to facilitate the development of treatments that are safe and effective for PWS, helping to advance potential treatments for PWS into clinical trials and beyond. We have spent hundreds, perhaps even thousands, of hours on the phone and in person, talking with companies about PWS, helping them understand PWS and the unmet needs of our community, facilitating clinical trial design and working to develop trials that have the best chances for success. We work with each company to de-risk the drug development process and speed up the timeline to taking a drug to the FDA for approval. We are committed to working with any company that has the potential to develop a medical product that might benefit individuals with PWS.
FPWR has been recognized for its productive collaboration with industry by the Clinical Trials Transformation Initiative, an organization comprised of more than 80 organizations from across the clinical trial enterprise with the mission to develop and drive adoption practices that will increase the quality and efficiency of clinical trials.
PWS is a complex rare disease that, as our community is well aware, is nuanced and not immediately understandable by people outside of our community. It is critical that the FDA fully understand how PWS impacts those with the disorder and their families so FPWR, PWSA |USA, and key leaders in the scientific community have held several meetings with the FDA to share the serious challenges of PWS and our loved ones’ unmet medical needs, and to bring the perspective of the PWS patients and caregivers to the FDA for their evaluation of new candidate drugs for PWS.
The FPWR team has also participated in meetings of pharmaceutical companies with FDA, providing the patient perspective in discussions about the development of specific medical products. In addition, FPWR Director of Research Programs, Theresa Strong has participated as a Panelist at meetings and webinars on how patients and patient groups can advance the patient perspective in drug development, review and approval:

Patient advocacy groups must rely on industry sponsors to submit New Drug Applications to the FDA. The New Drug Application (NDA) is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale in the US. Data gathered through clinical trials of the investigational new drug (IND) become part of the NDA. The drug sponsor is the ONLY entity that can submit an NDA to the FDA.
Drug sponsors control their relationship with the FDA and when to open communications. While patient organizations can help advise drug sponsors on a variety of areas relevant to patient perspective, which can impact their drug development and clinical trial program, we cannot dictate how the sponsor chooses to interact with the FDA or the decisions the sponsor makes. Typically, pharmaceutical companies maintain strict confidentiality about the details of their interactions with the FDA and many of the specifics of their submissions to the FDA. The FDA, by law, cannot discuss or disclose any information about their interactions with drug sponsors.
The timelines for new drug approvals are out of our hands. Many factors come into play when estimating a timeline for drug review and approval. Fortunately, many drugs in clinical trials for PWS have been given Orphan Drug Designations which speeds up the review of the NDA, however, before an application can be filed, multiple conversations need to take place between the drug sponsor and the FDA. These conversations are important steps in working towards a successful approval but we must realize, it typically takes a minimum of 3 months between each and every conversation. The more conversations that are necessary to fully review the drug, the longer the process will take.
.png?width=900&name=Visual%20Chart%20Page%20Iteration%205%20(3).png)
For more information on who can do what, watch this 6-minute presentation by Rob Lutz, PWSA | USA board member:
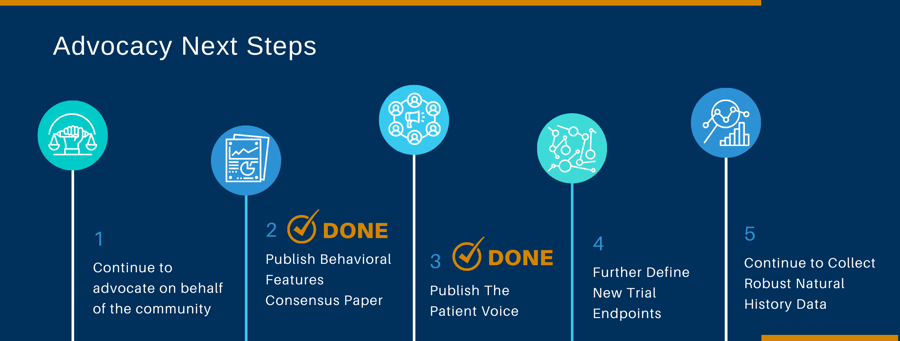
Until a CURE for PWS has been found, both FPWR and PWSA | USA will continue to advocate for the advancement of new, safe and effective drugs and devices to treat the challenges our loved ones with PWS face. We will work with all pharmaceutical companies that have products that might improve the lives of those with PWS.
For example, FPWR has circulated petitions among the PWS community to request that the FDA apply regulatory flexibility and engage in further dialogue with the PWS community regarding drug development for PWS. We will continue to provide opportunities for the patient community to share their experience with the FDA.
The FDA, as a general standard, requires positive results in 2 controlled studies or 1 well-controlled study with confirmatory evidence, such as natural history data. The Global PWS Registry is currently collecting natural history data that may support PWS clinical trials. We need the members of the PWS community to continue to complete surveys in the Registry so that our data reflects the entire PWS population.
Establishing additional clinical trial endpoints that accurately define what a positive result in PWS patients looks like is imperative to successful clinical trials. Clinical significance of the treatment effect and impact on aspects of PWS, other than hyperphagia, such as overall quality of life and the functioning of the participant, have not been fully explored and some interventions may positively impact different, or additional, aspects of the disorder including physical, cognitive and behavioral symptoms, which are not captured by current outcome measures. The PWS-CTC will continue to develop and evaluate new endpoints for PWS clinical trials.
-1.png?width=900&name=PWS%20Advocacy%20Activities%20(1)-1.png)
The Global PWS Registry is only as powerful as the people who participate. And learning more about the experiences, medications, symptoms, milestones, and other aspects of PWS is key to advancing our understanding and discovering new therapies and treatments.
Visit the Global PWS Registry for more information for researchers and patients. From there, register and be part of the global community working toward brighter tomorrows for our loved ones with PWS.
The Foundation for Prader-Willi Research (federal tax id 31-1763110) is a nonprofit corporation with federal tax exempt status as a public charity under section 501(c)(3).



The mission of FPWR is to eliminate the challenges of Prader-Willi syndrome through the advancement of research and therapeutic development.
Copyright © 2020. All Rights Reserved. Terms of Use. Privacy Policy. Copyright Infringement Policy. Disclosure Statement.