
The Global PWS Registry is a powerful tool for the PWS community to advance understanding of PWS, areas of unmet need, standards of care, and new therapies. Data from the registry is shared back to the PWS community and is also used by researchers and scientists. By participating, families of those with PWS become part of the research team, helping uncover trends in causes and diagnosis as well as new directions in therapies and treatment.
This page explains why the Global PWS Registry is so important, how to get involved, and what participation can mean for the future of people with PWS.
The Global PWS Registry is a comprehensive and secure database, compliant with U.S. Health information privacy laws and FDA regulations.
It was developed in 2015 to accelerate research and cures for the rare disease PWS. It works by creating a platform for patients around the world to share information about PWS on developmental history, medical complications, and quality of life.
By providing a means to collect and document the experiences, medications, symptoms, milestones, and other aspects of PWS from thousands of registry participants, the Global PWS Registry empowers PWS stakeholders to:
The Global PWS Registry was developed based on the current standards for patient registries. The registry questions, protocol, and data use policies have been reviewed and approved by an independent ethics committee called an Institutional Review Board (IRB), and data is collected only after participants provide informed consent.
Based on this, data collected can be fully utilized by university, government, and industry researchers (e.g. NIH, FDA) and published in major medical journals. The procedure for matching participants with clinical trials has been reviewed and approved by the IRB, facilitating patient recruitment. Finally, the registry information is maintained in a secure manner, compliant with patient privacy laws.


The Global PWS Registry provides a comprehensive database of individuals with PWS, which helps speed the recruitment process for clinical trials, guide the development of standards of care, and prioritize areas of unmet need. It also captures the full global diversity of PWS by including characteristics such as age, socioeconomic status, geography, ethnicity, living conditions, and access to care.
In her presentation on the Global PWS Registry, Jessica Bohonowych, PhD, used a quilt analogy to explain the importance of every individual's involvement with the registry.
“If you think of a beautiful, intricate story quilt. It’s made up of hundreds of little panels. Every single one of you is a panel of that quilt.” — Jessica Bohonowych, PhD
Further, in order to fully understand what treatment efforts are being made, what’s working, and what isn’t, she said, “We need every single story, every single patchwork on that quilt.”
Hear Dr. Bohonowych talk about the Global PWS Registry and how it’s accelerating PWS research and clinical trials.
This “quilt of information” is so important because PWS is considered a rare disease, occurring in approximately one out of every 15,000 births. PWS is recognized as the most common genetic cause of life-threatening childhood obesity. There is currently no cure.
Based on this, collecting, organizing and sharing such information on rare diseases, including PWS, is critical to increasing our understanding.
The Global PWS Registry is hosted on the NORD “IAMRARE” Registry Platform. NORD built its registry program with input from patients and patient organizations, researchers, and regulators as part of its mission to identify and treat all 7,000 rare diseases.
“We are committed to helping ensure that every rare disease has adequate natural history information,” said former NORD Director of Information Technology Vincent Tsugranes, “and that all rare disease patient organizations have access to the tools they need to advance patient-centered research.”
Essentially, to explore new therapies and treatments for PWS, the Global PWS Registry is vital. Thousands of people participate, and as more and more data accumulates, the registry will only get more powerful. This infographic illustrates how the registry works and why participation is so important.

The registry provides a repository for personal information and a way to support researchers. It captures insights from other respondents, provides a connection to clinical trials, and functions as a “living” registry as participants have the ability to update and refresh their information regularly.
Repository for information. The registry provides a way to capture information about people with PWS at the point they are in the PWS journey. As such, the registry is a valuable tool for participants to reflect on their status, symptoms, and progress.
“I filled out the initial surveys and it was helpful to look at different areas (neurological, medications, etc.) and systematically think through how my son is affected by PWS. I recently updated the surveys that, originally, I filled out in 2015. As I progressed through it, I was pleasantly surprised that while some issues have worsened, most were the same or improved. The registry is a great way for me to track my son’s progress.” — Ali Shenk, Mother of a Global PWS Registry Participant
Researcher support. The registry saves researchers valuable time by having the information already there to access, instead of having to start completely from scratch each time.
“If even one step in the development of a clinical trial, for instance, is saved because of readily available information in the registry, that’s priceless! ... Raising our kids takes a village and advancing research and care for those with PWS takes a village too! Our job, as parents in the PWS community, is to provide the information necessary for our village to move forward.” — Ali Shenk, Mother of a Global PWS Registry Participant
Insights from others with PWS. Through the registry, the PWS community is gaining valuable insights into diagnosis and treatments, developmental milestones, and how others are affected by PWS broadly speaking. Participants can use the registry to see de-identified data from other respondents, and de-identified data is being used to share useful information with the broader PWS community. For example, these publications and blogs with infographics highlight some of the many findings from the registry:
Publications:
Blogs with infographics:

Connection with clinical trials. The registry also helps match participants with potential clinical trials. If a participant’s profile matches the needs of an upcoming clinical trial, the registry will notify the participant and provide contact information for the study coordinator. Participants then have the option to follow up with the study coordinator and determine if the study is the right fit.
A living registry. Another benefit of the registry is that the collected data is not stagnant. The registry is designed to be a dynamic resource for the global PWS community. As such, the registry aims to be a Natural History Study, capturing the life experience of PWS from birth through one’s entire life. New questions are added as the community identifies new research areas. And, as more people participate, the data is de-identified and aggregated. Further, respondents have the ability to update their information as often as they need to. Surveys can be retaken as the person with PWS experiences changes in symptoms and life experiences. Participants are encouraged to update the registry once a year or after something has happened, such as a surgery or medical event. (Reminders to update the registry are sent once a year, based on preferences that participants select.)
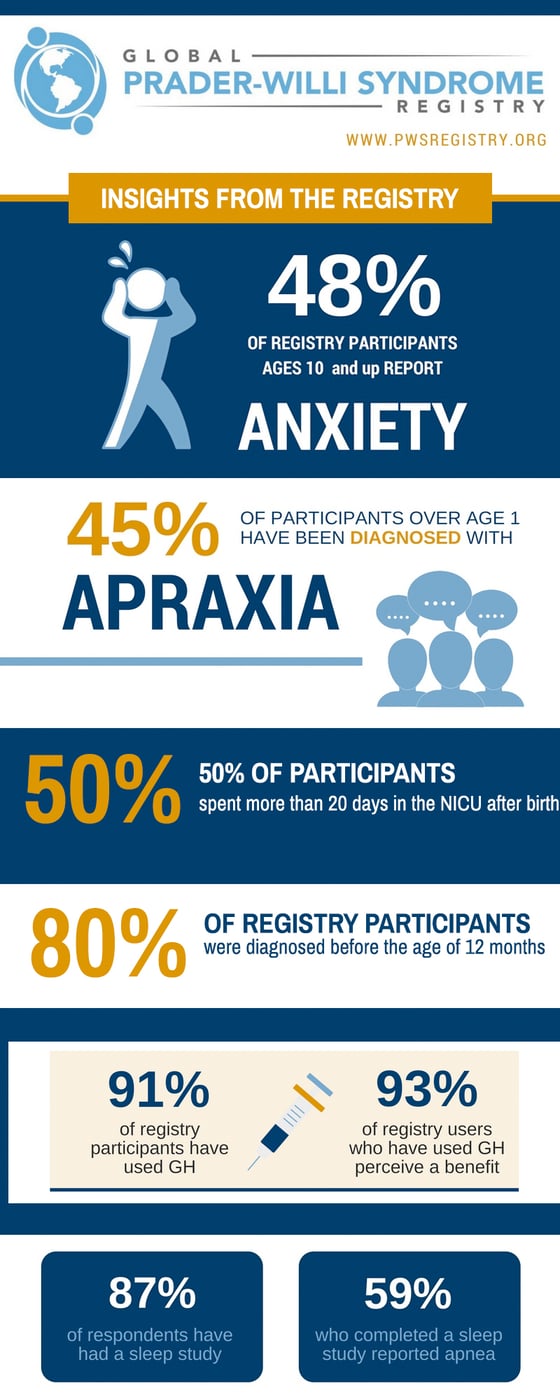
The Global PWS Registry consists of annual electronic surveys that collect information about the patient experience. The information is made anonymous and may be shared with individuals or institutions conducting research or clinical trials. Research studies are reviewed and approved by registry’s governing board that includes scientists, doctors, and parent advocates.
The initial registration and general medical history takes 20 to 30 minutes to complete. After that process, the registry leads to a list of forms on different aspects of PWS (e.g., behavior, scoliosis, medications). Depending on how many complications the person with PWS has experienced, this section may take 30 minutes to 2 hours to complete.
Entry of information into the registry does not have to be finished in one sitting. The data entered can be stored and completed at a later time.
With such a dynamic and comprehensive database involving personal information, addressing security is essential. Because the Global PWS Registry is hosted on the NORD platform, it was developed with extensive input from experts at the National Institutes of Health, patient advocacy groups, and the FDA. The Global PWS Registry is a secure platform, compliant with:
The Global PWS Registry guidelines and procedures have been reviewed and approved by an IRB (Hummingbird IRB), which has ensured that human subject research is conducted in accordance with all federal, institutional, and ethical guidelines.
Further, the information participants provide is made anonymous (de-identified) and then aggregated (combined and summarized) with data from other registry participants. The registry shares only de-identified data with the PWS community, PWS groups, investigators and institutions conducting research studies or clinical trials, companies developing potential drugs or other treatments for PWS, or other parties. All data sharing is in accordance with the data access and sharing guidelines outlined in the IRB-approved registry protocol. Any information that identifies participants is removed. The only people with direct access to the registry data are the co-investigators on the registry protocol.
This registry is open to all individuals with PWS. In addition, it can be completed by the parent or guardian of the person with PWS, or by the person with PWS if they are able.
To register, the first step is to read the terms and conditions and then create an account with the name of the “reporter or responder.” In creating an account there is an opportunity to add a participant. This is where the name and information of the person who has PWS can be listed.
From there, participants are directed to the Informed Consent document, which contains detailed information on how registry data will be collected, stored, and used. Once participants are comfortable with all the terms of the consent document, they can electronically sign it and proceed to answer the registry questions. Participants who have already signed the consent document can log in directly.
Next, participants are asked to complete the “Getting Started Survey,” which asks for the age and gender of the individual with PWS. These answers guide some of the questions throughout the other surveys. After this point, directions follow on completing and submitting the registration.
The Global PWS Registry is only as powerful as the people who participate. And learning more about the experiences, medications, symptoms, milestones, and other aspects of PWS is key to advancing our understanding and discovering new therapies and treatments.
Visit the Global PWS Registry for more information for researchers and patients. From there, register and be part of the global community working toward brighter tomorrows for our loved ones with PWS.

The Foundation for Prader-Willi Research (federal tax id 31-1763110) is a nonprofit corporation with federal tax exempt status as a public charity under section 501(c)(3).



The mission of FPWR is to eliminate the challenges of Prader-Willi syndrome through the advancement of research and therapeutic development.
Copyright © 2020. All Rights Reserved. Terms of Use. Privacy Policy. Copyright Infringement Policy. Disclosure Statement.