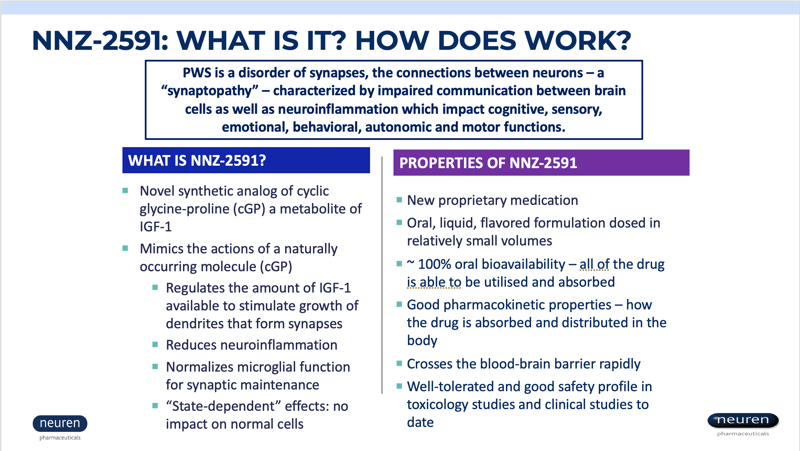
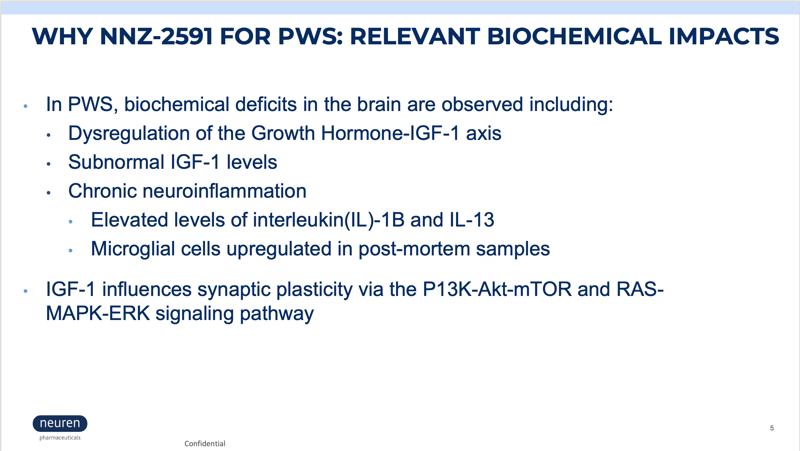
Neuren Pharmaceuticals is now enrolling children with PWS in a phase 2 clinical trial to measure the safety and efficacy of NNZ-2591. NNZ-2591 is a novel synthetic analog of cyclic glycine-proline (cGP), a metabolite of IGF-1. NNZ-2951 regulates the amount of IGF-1 available to stimulate the growth of dendrites that form synapses, reduces neuroinflammation, and normalizes microglial function.
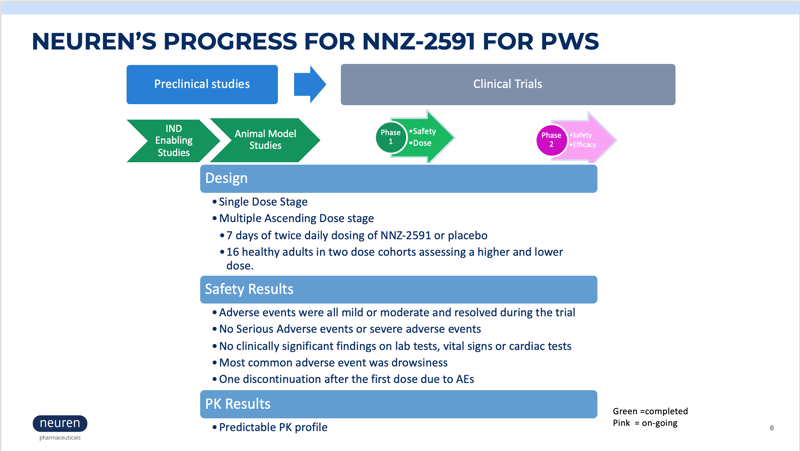
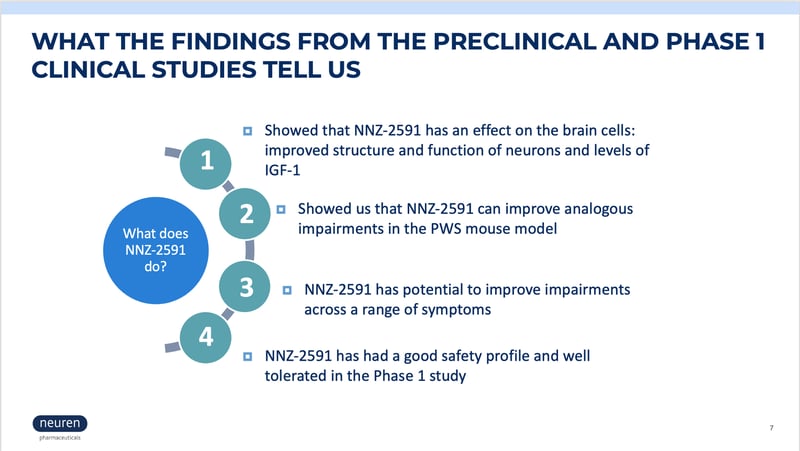
A live webinar was conducted on March 19th, 2024, that explains more about NNZ-2591 and provides details of the phase 2 study, including what you can expect if you choose to participate and the eligibility criteria for participation. Watch the complete webinar in the video below, or scroll down for slides from the presentation.
NNZ-2591 as a Potential Treatment for Prader-Willi Syndrome Presentation


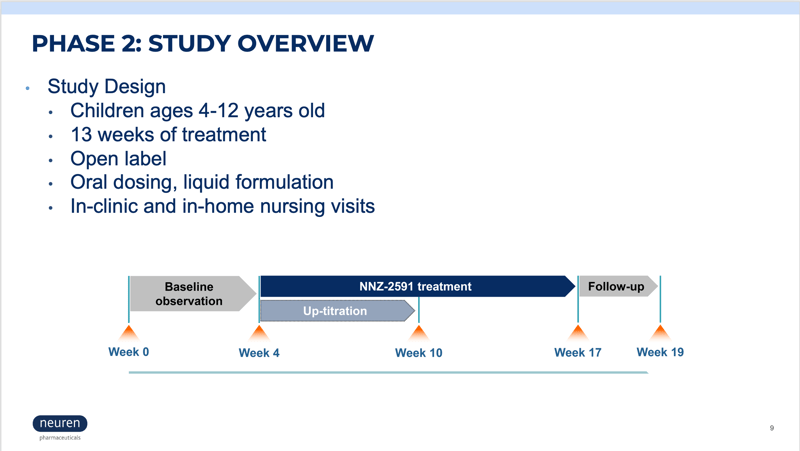

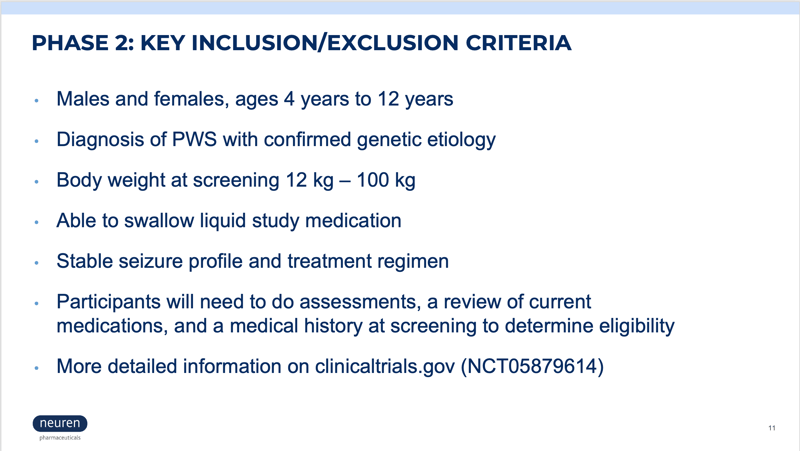



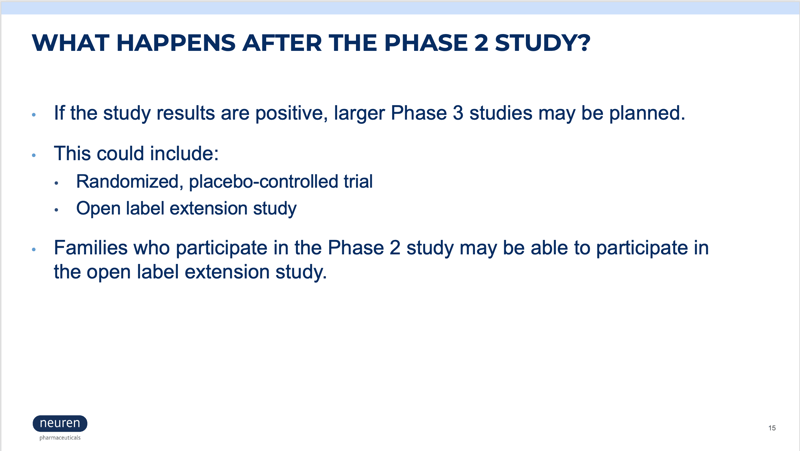
For trial questions and inquiries, please email PWStrialreferral@precisionformedicine.com







