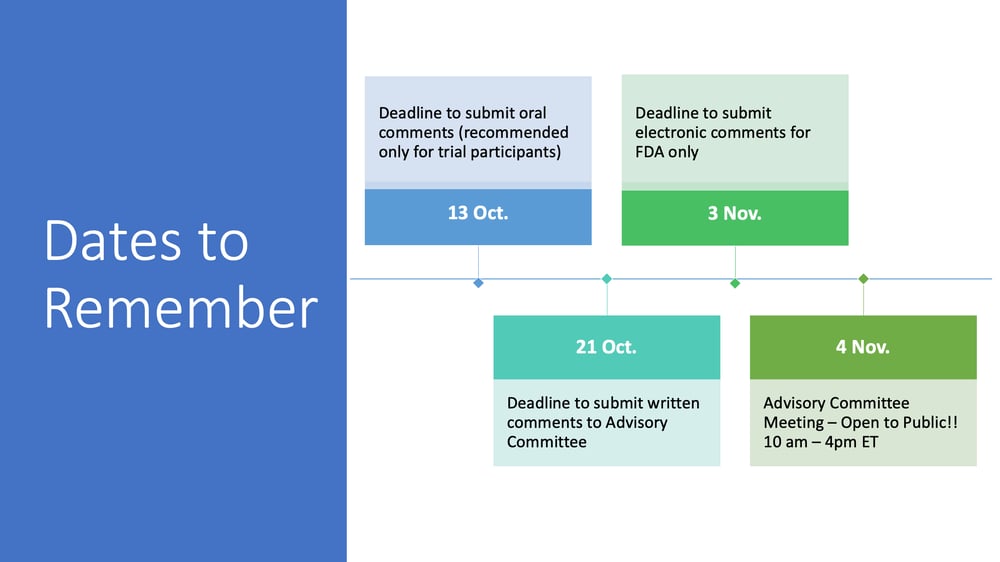
Would you like to be part of our community-wide effort to advocate for new treatments for PWS? The FDA is now accepting comments from the public regarding a new drug application currently under review for LV-101 (intranasal carbetocin), a potential treatment for PWS. Comments submitted by October 21, 2021, will be reviewed by the FDA Advisory Committee and will be considered when members of the Committee provide a recommendation to the FDA at the end of their public Advisory Committee meeting on November 4, 2021.

Advisory Committee meetings are held at the discretion of the FDA. Not all drug reviews include an Advisory Committee meeting. The meeting provides an opportunity for the FDA to get additional input from outside experts about the merits / concerns of a drug being considered for approval. These meetings also provide the public with an opportunity to hear about the data supporting drug approval from the company sponsor and hear from the FDA about their assessment of the safety and efficacy of the drug. This forum also allows the public an opportunity to comment on the medical needs of the patient community and the potential for the new drug to address those needs. The FDA is not required to follow the recommendations of the Advisory Committee, but it usually does.
Our goal is to provide the Advisory Committee, and the FDA, with patient / family perspectives regarding this treatment for PWS and our desire to have the treatment approved. To allow the Advisory Committee to focus on the most relevant comments, we suggest that the most impactful comments will come from those who participated in the CARE-PWS clinical trial, and those who live every day with PWS.
We are at a critical moment in PWS history. Individuals with PWS and their families WILL BE IMPACTED by the Committee’s decision! Your thoughtfully constructed comments will help the Advisory Committee better understand the challenges of PWS as well as your perspective on how this drug might make a meaningful impact for people with PWS. Your words should be your own, but to help you construct your comments, we have provided a few topics that the FDA seeks the patient perspective on. You do not need to comment on every point listed below, but your specific examples and perspectives on any of these points can help the Advisory Committee put the risks and benefits of LV-101 into context.
- The unmet medical need in PWS: PWS has a tremendous impact on the person with PWS and their family. It is a serious, life-threatening disorder. Among its many symptoms, families consistently indicate that hyperphagia and anxiousness are two of the most challenging aspects of PWS, and that there are currently no therapies that adequately address these needs. Comments about how these features of PWS impact the ability of the person to live a healthy and fulfilling life will help the Advisory Committee understand the problem.
- Even a modest improvement for PWS is impactful: Modest improvements in key behaviors (such as hyperphagia or anxiety) will have a meaningful impact on the ability of the person with PWS to [explain what your loved one may be able to accomplish with a treatment, how daily living might improve, as well as the ability to reach long term goals]
- We are willing to accept risk: It is important for the Advisory Committee to consider that our kids live with risk every day (e.g., individuals with PWS sometimes run away to get food, or binge eat if they get access to food, etc.). In addition, many families struggle to manage PWS–associated behaviors using medications that have NOT been tested in the PWS population. We recognize that any new medication has risks, but LV-101 appears to have a favorable safety profile, and any risk should be considered in the context of existing risk.
- We are willing to accept the uncertainty of BENEFIT: We expect that not every drug will help EVERY person with PWS. LV-101 (carbetocin) has a favorable safety profile, and we’d like to be able to work with our doctors to see how it works in our children.
- Additional trials are an undue burden and are not realistic. The FDA has previously suggested the additional clinical trials are needed. However, the challenges of PWS, as well as our small patient population, makes participating in and completing a clinical trial incredibly difficult.
For the best readability, submit comments via PDF or MS Word file
and NOT through the text field option.
Did your loved one participate in the CARE–PWS trial?
If your loved one participated in the CARE–PWS trial of LV-101 (carbetocin), you may have additional observations that could be meaningful to the FDA and the advisory committee. Below are a few points you may want to consider as you draft your comments (note that you may want to highlight very early in your comments that your loved one with PWS was a CARE-PWS trial participant):
- How has LV-101 changed your child’s relationship with food? Describe what your child’s food interest and hunger looked like before treatment. Could they delay meals or snacks? Could they be a part of events where food was available? Did they talk or ask about food? How is it different now with treatment?
- Describe your child’s anxiety before beginning treatment. What did it look like? Tearful, angry, irritable, aggressive? What triggered it? How are things different now with treatment?
- Was your child rigid prior to beginning treatment? What about now?
- What is the most important way LV-101 has changed in your life? What has been the most important change in your child’s life?
Three Ways To Prepare For An Impactful Statement
1. Watch our Webinar
It is important that the FDA hears personal stories of how this drug has benefitted the person with PWS and their family and we strongly encourage EVERYONE who has experience on the drug to submit a THOUGHTFUL comment to the advisory committee for their consideration. To provide additional support to our community, we recently conducted an advocacy webinar. In the recorded presentation, we answer questions including:
- What is an Advisory Committee Meeting?
- What information is considered during a drug review?
- How can the PWS community impact the FDA's decision?
- Important dates for submitting comments to the FDA
We encourage members of our community to learn more about this exciting opportunity by watching the webinar and submitting written comments to the FDA. Watch the presentation today:
2. Download the Slide Deck
Download the presentation Advocating for New Treatments for Prader-Willi Syndrome here
3. Read the Meeting Announcement
Read the FDA Advisory Committee meeting announcement here
For the best readability, submit comments via PDF or MS Word file
and NOT through the text field option.