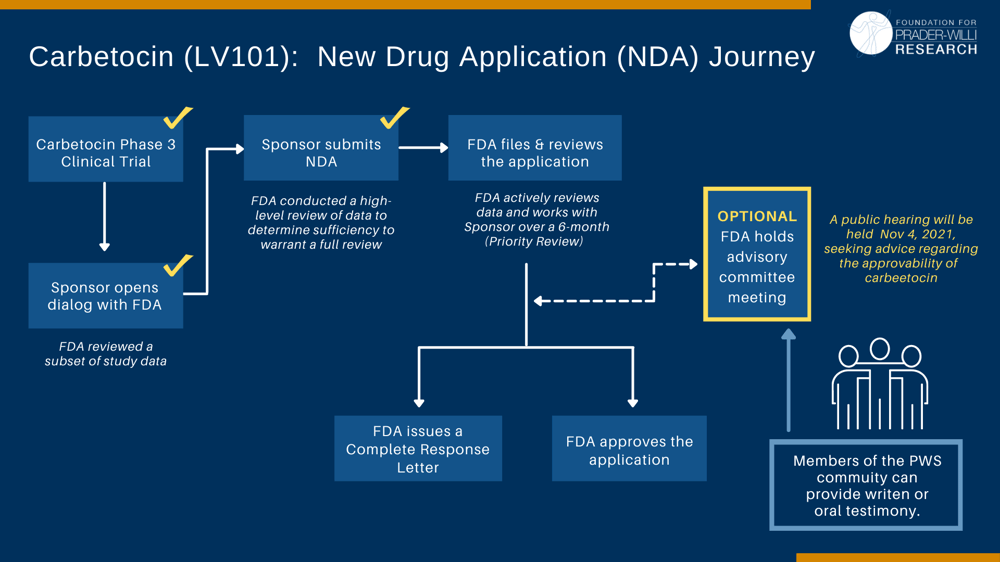
Levo Therapeutic’s New Drug Application (NDA) for carbetocin as a treatment for PWS has been scheduled for a public meeting of the Psychopharmacologic Drugs Advisory Committee to be held on November 4th, 2021. The Food and Drug Administration (FDA) calls on Advisory Committees to provide independent advice that contributes to the agency's regulatory decision-making. Interested community members are encouraged to share their views with the Advisory Committee.
This is a critical step in the drug approval process and provides the PWS community an opportunity to speak directly to the FDA regarding the benefit Carbetocin can provide to our community.
- The advisory committee meeting will take place virtually through an online teleconference on November 4, 2021, from 10 a.m. to 4 p.m. Eastern Time. See the meeting announcement here.
- A webinar was recorded to help community members learn how to make the greatest impact in providing comments for the advisory committee. Scroll down to watch the recorded webinar.
- Public comments to the Advisory Committee may be submitted on or before November 3rd, 2021. Visit https://www.regulations.gov to learn more or click here to submit your comment. [Docket No. FDA-2021-N-0860] Comments received on or before October 21, 2021, will be provided to the Advisory Committee. Comments received after that date will be taken into consideration by FDA.

What is an Advisory Committee?
Advisory committees provide independent advice and recommendations to the Food and Drug Administration (FDA) on scientific and technical matters related to the development and evaluation of products regulated by the Agency. Although the committees provide recommendations to the Agency, final decisions are made by FDA
Advisory committee meetings can occur during any stage of a medical products review process and after a product has been approved and marketed. Typically, an advisory committee meeting is held to assist the review division with interpretation when questions or difficulties related to trial data arise.
What will happen during the Advisory Committee meeting?
During the public meeting, the company sponsor presents its data, the FDA scientists present their review, and members of the public are invited to speak briefly during the “open public comment period.” Advisory committee members ask questions of the sponsor, the FDA, and occasionally individuals who speak during the open public comment period. At most advisory committee meetings, most of the research-based presentations are by the company and its paid consultants, with less time for presentations by FDA scientists. Outside experts, such as government researchers or independently funded researchers, are sometimes invited to make formal presentations at an advisory committee meeting, but such presentations are not typical.
After reviewing the data and listening to the company presentation, the FDA presentation, and any public comments, committee members discuss and vote on questions that the FDA has prepared and provided to committee members in advance. The prepared questions for new medical products include whether the product is effective, whether it is safe, and “whether the safety and effectiveness information submitted for a new drug is adequate for marketing approval.”
Who can attend an Advisory Committee meeting?
Advisory Committee meetings are public and open to anyone who wishes to attend. This Advisory Committee meeting will be held via online teleconference.
How can you participate in the Advisory Committee meeting?
Community members may provide written comments or request an oral presentation.
- If you are a PWS community member but did not participate in the Carbetocin clinical trial, we encourage you to submit a written comment to the advisory committee.
- If your family has participated in the Carbetocin clinical trial, you may be interested in sharing your experience with the committee through a short, oral testimony. We encourage community members interested in oral testimony to please contact Susan Hedstrom at Hedstrom@FPWR.org or Paige Rivard at PRivard@pwsausa.org.
Speaking time is very limited (1 hour). Depending on how many individuals sign up to speak, allotted times may range from 2-5 minutes. An interested person who wishes to make an oral presentation during the Open Public Hearing portion of an advisory committee meeting should register with FDA before the meeting. Note that because speaking time is so limited, we encourage community members who do not have direct experience with carbetocin to submit written comments rather than request a speaking slot.
Preparing for the Greatest Impact
Learn more about how YOU can make the most of this exciting opportunity by watching the recording of our webinar presented Oct. 8, 2021. Stream the video below:







