 A new study on the role of Magel2 in feeding and energy helps define the underlying biology of ‘the problem’ in appetite and energy balance brain circuits in PWS. It also suggests that therapeutic approaches to strengthen the anorexigenic circuitry and enhance the function of POMC neurons might serve to tip the balance of hyperphagia, hunger and satiety back towards normal in PWS.
A new study on the role of Magel2 in feeding and energy helps define the underlying biology of ‘the problem’ in appetite and energy balance brain circuits in PWS. It also suggests that therapeutic approaches to strengthen the anorexigenic circuitry and enhance the function of POMC neurons might serve to tip the balance of hyperphagia, hunger and satiety back towards normal in PWS.
In virtually all individuals with PWS, the MAGEL2 gene is missing or inactivated in the PWS critical region on chromosome 15. Although MAGEL2 is not the only gene disrupted in PWS, its loss likely significantly contributes to the characteristics of PWS. Mice missing the Magel2 show some of the major features of PWS (decreased activity levels, slow metabolism, increased fat mass, decreased muscle mass, infertility). In addition, disruption of only MAGEL2 in humans is associated with a recognizable syndrome that overlaps many symptoms with PWS.
This syndrome, called Schaaf-Yang syndrome (SYS) includes feeding difficulties and hypotonia in infants, intellectual disability, autism, and behavioral difficulties. SYS was first identified a couple of years ago, and the extent of overlap between PWS and SYS is currently being investigated by Dr. Schaaf at Baylor College of Medicine in an FPWR funded project titled: The MAGEL2 phenotype in comparison to classic Prader-Willi syndrome. It is critically important to understand the normal function of the MAGEL2 gene in order to understand its contributions to the PWS phenotype (characteristics), and to determine if it is a good target for therapeutic development to recover/replace loss of Magel2 function.
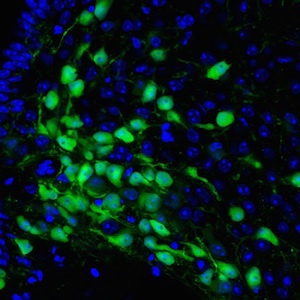
With support from FPWR, Dr. Sebastian Bouret’s lab at Children's Hospital of Los Angeles (CHLA) has been studying how Magel2 acts in the developing brain to influence the circuits that control feeding and energy balance. Detail on the project can be found here. In a paper just published in the journal Human Molecular Genetics, and highlighted in a CHLA press release, Dr. Bouret's group shows that when MAGEL2 is lost, as in PWS, the brain circuits that are sent the "I'm full" message do not normally develop. These circuits are scientifically called anorexigenic and are characterized by POMC neurons. In contrast the "I'm hungry" circuits, called orexigenic, develop as expected.
Mechanistically, it appears that Magel2 normally functions to nurture the POMC neurons, helping the outgrowth of “neurites”, or spidery projections that connect neurons and allow them to work together. When Magel2 is missing, the POMC neurons fail to mature and connect as they should, compromising the anorexigenic circuits.








