
Genetic therapy is an approach to using or modifying genes to treat, cure, or prevent a disease or medical condition. There are several potential gene therapy strategies that are being developed to treat disorders:
Genetic therapy can also include modifying the epigenome, the chemical modifications that determine if a gene is active or silent. Genetic therapies are still in relatively early stages of development and are being studied to make sure they are safe. Currently, gene therapy is only being tested in diseases that don’t have any other cures. Genetic therapies are described on the NIH website here.
In 2019, FPWR invited genetic researchers Drs. Stormy Chamberlain and James Resnick to give an informal presentation to discuss with the PWS community the state of genetic therapy for PWS and explain the challenges and the strategies being used to explore the potential of genetic therapy for PWS. You can view the presentation below:
Genetic therapies have the potential to provide transformative treatments in PWS, but much is still unknown about the feasibility of such a treatment. Gene therapy strategies are attractive because they seek to directly correct the underlying issue in PWS – the lack of expression of several critical genes in the PWS region of chromosome 15. In theory, such an approach might simultaneously improve many aspects of PWS, but much still needs to be learned, and tested, to determine what benefits this approach will have for individuals with PWS.

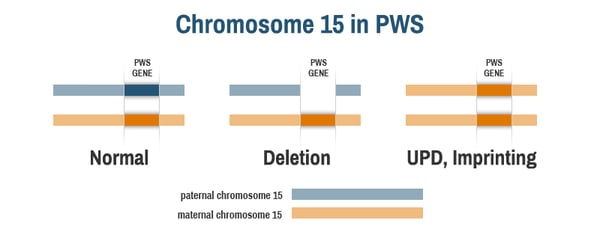
To understand how genetic therapy for PWS might work, it’s first important to know that the PWS region of chromosome 15 is imprinted, meaning that the genes behave differently depending on whether they were inherited from a person’s mother or father. In people without PWS, each cell has one copy of chromosome 15 inherited from their mother (the maternal chromosome, in orange below) and one copy of chromosome 15 inherited from their father (the paternal chromosome, in blue below). The PWS genes on chromosome 15 are only active on the paternal chromosome; they are inactive, or silent, on the maternal chromosome.
In people with PWS, the active, paternal copy of chromosome 15 is missing. Those with PWS by deletion have the full maternal chromosome 15, but the PWS genes are deleted on the paternal chromosome 15 (middle image). Those with PWS by uniparental disomy (UPD) or due to an imprinting defect have two copies of the maternal chromosome 15, but no paternal chromosome 15 (right image). Notably, all people with PWS have at least one copy of the maternally inherited chromosome 15. The PWS genes are present on this maternal chromosome, but they are inactive, or “silent.”
There are currently two general genetic therapy approaches being evaluated for the treatment of PWS:
Some of the possible strategies currently being investigated to achieve genetic therapy in PWS are described below, but importantly, an initial proof-of-concept study, funded by FPWR, has shown that genetic therapy might positively impact characteristics of PWS.
At this time, we do not know when the best time to introduce a potential gene therapy would be (e.g., infancy, childhood, or adulthood). Current research efforts include studies that will help scientists determine what benefit might be expected from doing gene therapy at different developmental stages.
Gene activation and gene replacement approaches for PWS would generally work similarly for PWS by deletion, UPD, and imprinting defects. However, there may be some differences in the effects of gene therapy in the different genetic subtypes. This is because there are two chromosome 15’s present in individuals with PWS by UPD or imprinting mutation, while there is only one set of PWS genes in individuals with PWS by deletion. Also, turning “on” some genes on chromosome 15 may result in other genes turning off. So “fine tuning” the level of PWS gene expression may be important, and there may be differences between the genetic subtypes. Studies in cells from individuals with PWS (deletion, UPD, and imprinting defect), and different mouse models of PWS will help scientists sort through the intricacies of how best to achieve genetic therapy in all subtypes of PWS.
We are fortunate to live in a time when gene therapy is progressing rapidly to the clinic for many genetic and acquired disorders. Lessons learned from genetic therapy research for Fragile X syndrome, Angelman syndrome, and other syndromes are helping smooth the path for genetic therapy for PWS, although of course each syndrome is unique and poses different challenges.
There are many questions that need to be answered before genetic therapy becomes a feasible treatment for PWS. Scientists will need to determine if this therapy is likely to be beneficial for patients, and if there are any safety concerns. They will need to develop methods to measure if the genetic therapy is working. Among the feasibility questions that need to be addressed prior to trying genetic therapy in individuals with PWS:

Finally, there is a significant difference between successfully doing this therapy in cell and mouse models and successfully doing this therapy in humans.
There are several approaches that FPWR-funded researchers are taking toward gene therapy.
Dr. Stormy Chamberlain studied PWS, Angelman, and Dup15q syndromes using stem cells from patients. These cells are an important tool because they can be turned into any cell type in a laboratory dish, including brain cells (neurons). Since PWS is primarily a neurodevelopmental (brain) disorder, this research allowed her to study PWS stem cells that have been turned into neurons and look at PWS gene regulation in those neurons. Her lab focused much of their research on understanding how the PWS genes on the maternal chromosome 15 become silenced and how they might be able to “awaken” or reactivate them. FPWR-Canada funded a gene therapy grant to Dr. Chamberlain and Dr. Marc Lalande titled “Therapeutic Potential of Blocking Zinc Finger Protein 274 Binding to the PWS Locus.” This project led to the reversal of PWS in neurons in a dish.

Dr. Marnie Blewitt from the Walter + Eliza Hall Research Institute in Australia is also looking at a potential gene therapy for PWS and Schaaf-Yang syndrome (SYS). Dr. Blewitt has found that the protein SMCHD1 is involved in silencing PWS-region genes on the maternal chromosome. Blocking SMCHD1 can cause the PWS-region genes on the maternal chromosome to “wake up” and become active. Her research uses stem cells from people with PWS and SYS to see if removing SMCHD1 in those cells will allow the PWS genes to become active, as an exciting proof-of-concept study for a potential gene therapy for PWS and SYS. This project was initially funded by FPWR and the Prader-Willi Research Foundation of Australia, and is titled “Targeting SMCHD1 to Address the Underlying Cause of PWS and SYS.” You can learn more and watch an explanatory video here. Dr. Blewitt has recently received an additional $5 million investment from her institution to continue this research.
Drs. Charles Gersbach and Nahid Iglesias from Duke University have been working on potential genetic therapies for PWS and has shown that “epigenome editing” can reactivate the maternal genes in the PWS region in human cells. The current study will focus on determining the molecular requirements to permanently reactivate the maternal genes in the PWS region so that gene expression is maintained long-term in the cells, and the initial findings look promising. This project uses cutting-edge CRISPR technology. If successful, this approach from this project will lay the groundwork for the therapeutic development of a one-time gene therapy establishing stable reactivation of PWS genes for the lifetime of the patient.

As we know, hyperphagia (excessive hunger) and the associated metabolic changes are one of the greatest challenges that individuals with PWS and their families face on a daily basis. Dr. Lei Cao from The Ohio State University has developed a gene therapy that targets the metabolic roots of PWS within the brain’s center for energy regulation. This group has developed an approach using a single dose of a gene whose deficiency is associated with human obesity (brain-derived neurotrophic factor, or BDNF). This gene therapy is highly effective in animal models of obesity, and was recently tested in a PWS mouse model with encouraging results. In this funded project, Dr. Cao and her team will assess this novel gene therapy approach for metabolic dysregulation in a PWS mouse model.
Finally, one of the projects that has progressed the furthest is led by Dr. Yong-hui Jiang from Yale University. Dr. Jiang has been looking at small molecules that might activate PWS gene expression. From a previously funded project by FPWR, they found two different small molecule compounds (UNC638 and UNC0642) that can activate genes from the PWS region on the maternal chromosome 15 in human PWS cells. This is a very exciting step forward in potential gene therapy for PWS, which has been followed by an initial preclinical study to evaluate the feasibility of epigenetic therapy in PWS. This work is currently being carried forward with funding from the NIH.
Collectively, these projects and other work in the field will develop strategies for PWS genetic therapy and provide the rationale for bringing these approaches into testing in the clinic.
There are many ways you can help move this exciting research forward! Raising awareness for PWS is important, to make sure that all children with PWS are diagnosed as soon as possible so that they can benefit from future therapies. If you have a child or relative with PWS, consider enrolling them in studies to better understand PWS clinical features, and/or in clinical trials. This will help move research forward in PWS, even if it is not directly related to genetic therapy. Another way to help is to donate or fundraise for FPWR, because that money goes to help fund researchers who study PWS.
Advocating for general biomedical research is also helpful, because many researchers who get initial funding from FPWR may need follow-up funding from larger agencies (such as from the US National Institutes of Health [NIH]) to further PWS research. So talking to your representatives and advocating for national research funding is a great way to help. We can all work together for a better future for our loved ones with PWS.


The Foundation for Prader-Willi Research (federal tax id 31-1763110) is a nonprofit corporation with federal tax exempt status as a public charity under section 501(c)(3).



The mission of FPWR is to eliminate the challenges of Prader-Willi syndrome through the advancement of research and therapeutic development.
Copyright © 2020. All Rights Reserved. Terms of Use. Privacy Policy. Copyright Infringement Policy. Disclosure Statement.