FPWR
Recent Posts
Background In response to Acadia Pharmaceuticals’ announcement that the COMPASS-PWS Phase 3 trial of intranasal carbetocin did not meet its primary or secondary endpoints and would not advance further, the Foundation for Prader-Willi Research (FPWR) ...
Understanding the endocrine system is essential for managing Prader-Willi syndrome (PWS). From infancy through adulthood, hormone-related challenges shape growth, development, metabolism, and overall health. In this 2025 FPWR Conference presentation,...
Topics: Therapeutic Development, Research
A special blog contribution from our 2025 FPWR Art Auction Participants.
Topics: Stories of Hope
Clinical trials are the bridge between discovery and real-world treatments, and families in the Prader-Willi syndrome (PWS) community play a critical role in making them possible.
Topics: Research, Clinical Trials Opportunities
FPWR is dedicated to advancing research and accelerating the development of safe and effective treatments for people with Prader-Willi syndrome (PWS). While significant unmet needs remain, there are several therapies—both established and emerging—tha...
Soleno Therapeutics is committing up to $5 million to accelerate progress toward a cure for Prader-Willi syndrome by funding groundbreaking genetic research.
Families of children with Prader-Willi syndrome (PWS), your participation has the power to make a real difference—not only for your own child, but for the PWS community at large. Below are two research studies currently recruiting through the Neurode...
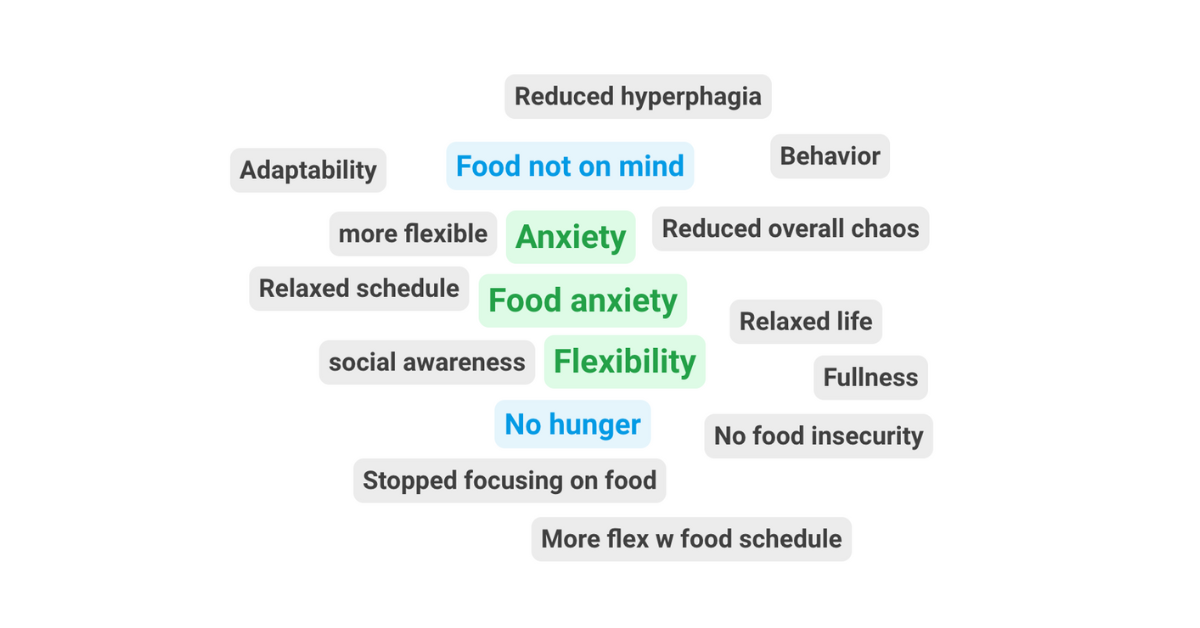
We are saddened to share disappointing news from Acadia Pharmaceuticals. The Phase 3 COMPASS clinical trial evaluating intranasal carbetocin (ACP-101) in individuals with PWS did not meet its primary endpoint of reducing hyperphagia. The study also s...
Adults with Prader-Willi syndrome (PWS) can experience earlier-onset changes in health and daily functioning. During a 55‑minute presentation at the United in Hope PWS conference, Dr. Laura de Graaff (Erasmus MC, Rotterdam) discussed practical steps ...
Topics: Adults