Topics: Research
Physical activity and exercise are an important part of care for individuals with PWS. However, this can be difficult due to poor muscle tone and orthopedic issues. Many individuals require bracing, casting, and or surgery for spinal issues. Here, we...
In this 1 hour and 2‑minute video, Dr. Theresa Strong, FWPR’s director of research programs, shares highlights from the 2021 PWS Research Symposium. The session includes Q&A from participants in the 2021 FPWR Virtual Conference.
Topics: Research
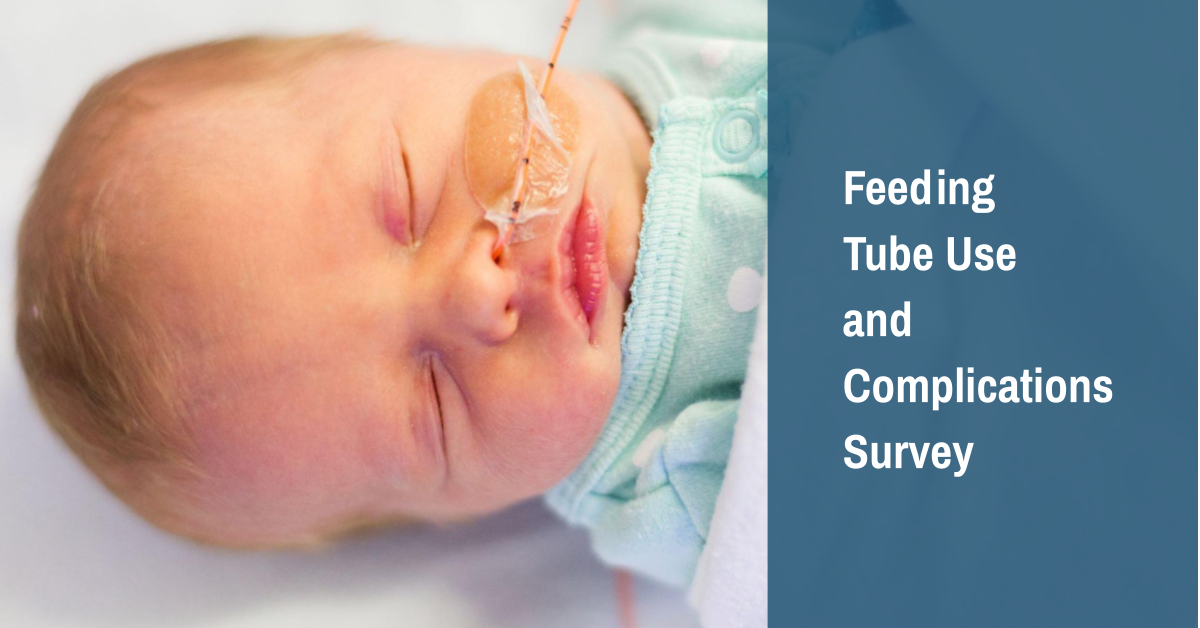
Researchers from the PWS CLIC (Clinical Investigation Collaborative) need your help to learn more about the use of feeding tubes in infants with PWS.
Topics: Research
With the support of the John C. Storr and Suzanne M. Storr Foundations, FPWR is excited to announce the launch of three new research programs designed to translate findings from the lab to human clinical trials, accelerating the development of new tr...
Topics: Research
In this 35‑minute video, Sara Cotter, founder and CEO of Levo Therapeutics, joins other members of the Levo team in describing the company’s mission of developing impactful therapies for the PWS community. The session includes Q&A from participan...
Topics: Research
Harmony Biosciences recently announced changes to their Phase 2 Clinical Trial of Pitolisant that reduce the requirements of participating in the study.
Topics: Research
A special contribution by guest blogger Jeannine Kowal Caitlin's birth was uneventful. She was induced at thirty‑nine and a half weeks because of growth concerns. Her Apgar score was 8/9, and we were discharged from the hospital two days later. We no...
Topics: Stories of Hope
In this one hour and 9‑minute video, Dr. Lauren Schwartz Roth, a clinical psychologist and parent to a young adult with PWS, explains how parents and caregivers can effectively assess and manage mental health issues if they arise in loved ones with P...
Topics: Research
ARD-101 is a new, oral therapeutic that has shown promising activity in reducing appetite and promoting weight loss in preclinical (animal model) studies.
Topics: Research