Today we received disappointing news from Millendo in regards to their Phase 2 study of Livoletide in PWS (Zephyr). Unfortunately, results from the trial do not show the significant improvements in hunger that we had all hoped to see and Millendo has...
In our latest podcast, Patrice Carroll, Director of PWS Services at Latham Centers in Cape Cod, shares how to how to help our loved ones with PWS through this unusual time of social distancing and isolation due to COVID-19. To listen, just click abov...
Topics: Research
COVID-19 has turned all of our lives upside down. These times can be particularly challenging for our loved ones with PWS who rely on routines and schedules. In this webinar, Elizabeth Roof, Senior Research Specialist at Vanderbilt Research Center, s...
Topics: News
In our latest podcast, Lauren Schwartz-Roth, clinical psychologist and mom to a young adult with PWS, shares tips on how to stay calm and manage anxiety during COVID-19. To listen, just click above, or read below for a list of tips to start using tod...
Topics: Research
You won’t want to miss these speakers at the 2020 Virtual PWS Family Conference, October 6–9. Inspiration, hope, people who get it — that’s the 2020 Virtual PWS Family Conference! At this free virtual conference, you can join like-minded game-changer...
Topics: News
Soleno therapeutics recently completed enrollment of their ongoing Phase III Clinical trial, DESTINY PWS, and is expecting to announce top-line data the first half of 2020. The study is evaluating once-daily Diazoxide Choline Controlled-Release (DCCR...
Topics: Research
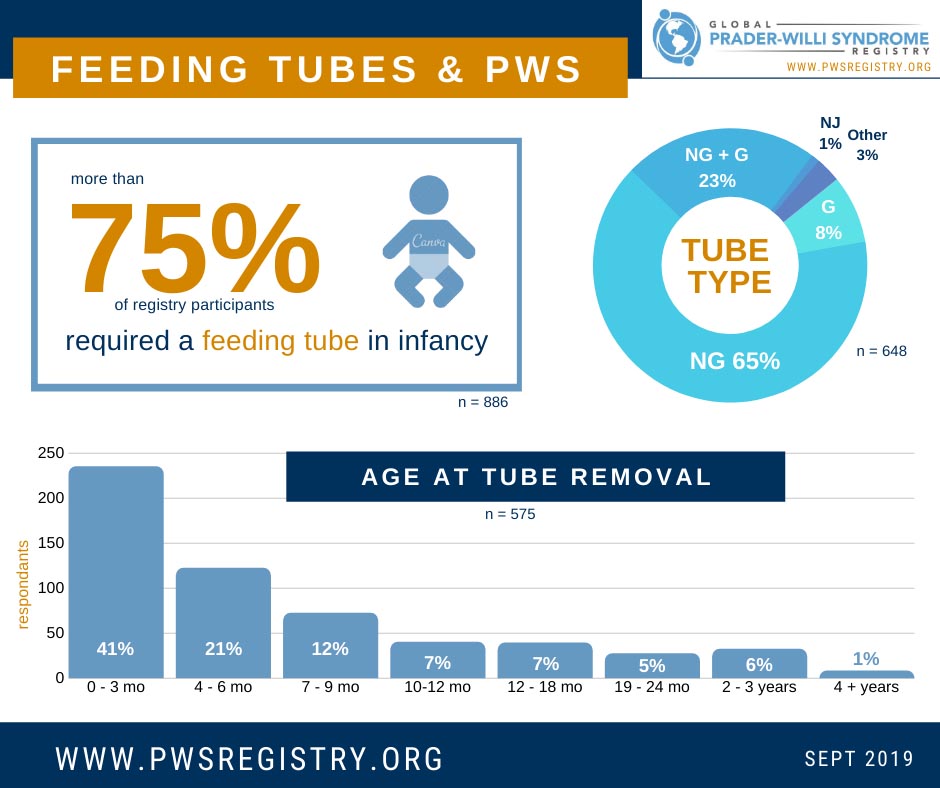
For parents of an infant with PWS, one of the first major challenges they face is feeding difficulties and having their baby consume enough nutrition and calories. Data from the Global PWS Registry shows that more than 75% of registry participants re...
Topics: Research
A special contribution by guest blogger Natalie Brenneman Evan emerged into the world lifeless and blue. I remember watching my husband shifting back and forth on his tiptoes behind the medical team working to resuscitate him. “It’s a boy!” he finall...
Topics: Stories of Hope
Temper outburst and disruptive behaviors are among the most challenging aspects of PWS, both for the individual with PWS and their family (Tsai 2018). Now, a promising study finds that four out of five participants had a reduction in temper outbursts...
Topics: Research