Topics: Parents
Caring for a child or adult with Prader-Willi syndrome (PWS) often means juggling medical appointments, behavior plans, daily routines, and the emotional ups and downs that come with them. Too often, caregivers put their own needs last — not because ...
As we wrap up 2025, we’re celebrating a year shaped by real progress… scientific breakthroughs, new treatment pathways, and meaningful improvements in care for people with Prader-Willi syndrome (PWS) and Schaaf-Yang syndrome (SYS).
Topics: News
Mark your calendars and join us October 9–10, 2026, as parents, caregivers, and medical professionals from across the country gather in Philadelphia, Pennsylvania for FPWR’s 2026 PWS Family Conference — a two-day event designed to connect, learn, and...
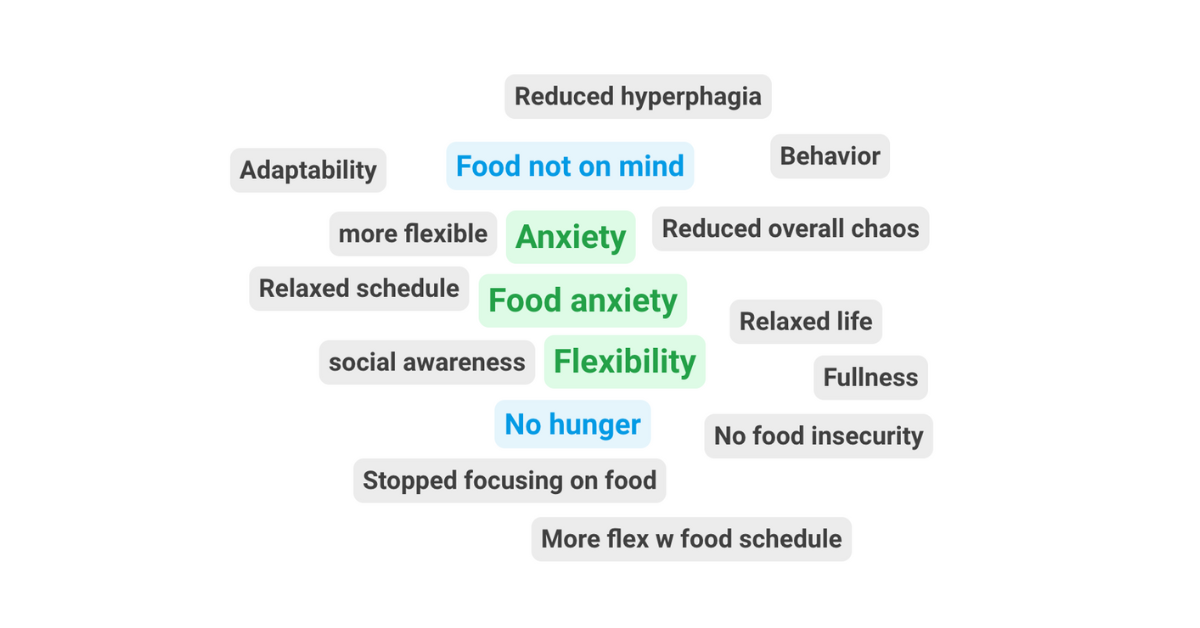
Behavioral challenges are among the most difficult aspects of Prader-Willi syndrome (PWS). These challenges affect the individual, family, school, and community. Anxiety, rigidity, and difficulty with transitions can make daily life unpredictable and...
We are pleased to announce the recipients of our second round of grants for 2025, totaling $925,117 in awards, as part of the Foundation for Prader-Willi Research’s (FPWR) ongoing commitment to advancing innovative research and bold initiatives in Pr...
Topics: Research, Schaaf-Yang Syndrome
Background In response to Acadia Pharmaceuticals’ announcement that the COMPASS-PWS Phase 3 trial of intranasal carbetocin did not meet its primary or secondary endpoints and would not advance further, the Foundation for Prader-Willi Research (FPWR) ...
Understanding the endocrine system is essential for managing Prader-Willi syndrome (PWS). From infancy through adulthood, hormone-related challenges shape growth, development, metabolism, and overall health. In this 2025 FPWR Conference presentation,...
Topics: Therapeutic Development, Research
A special blog contribution from our 2025 FPWR Art Auction Participants.
Topics: Stories of Hope
Clinical trials are the bridge between discovery and real-world treatments, and families in the Prader-Willi syndrome (PWS) community play a critical role in making them possible.
Topics: Research, Clinical Trials Opportunities